 The second part of this article analyzes the electrochemical noise factor in corrosion processes and presents tests carried out with their respective results in this class of pigments.
The second part of this article analyzes the electrochemical noise factor in corrosion processes and presents tests carried out with their respective results in this class of pigments.
Dr. Lars Kirmaier*
Electrochemical Noise Analysis: Electrochemical noise analysis (ECN) is a very sensitive method for recording local corrosion processes and changes in materials, and has been successfully used for many years in various applications [6], among others, in the field of coating testing [7]. Measurements to investigate the dissolution behavior of non-alloy steel in aqueous dispersions for coating were carried out without external current, using a three-electrode configuration. For this purpose, two macroscopically identical working electrodes made of C55 are short-circuited through a zero-resistance ammeter and connected to a high-strength potentiometer and an Ag/AgCl reference electrode.
The measured noise signals are filtered by means of a bandpass. This allows them to be separated from steady-state components (current and potential) and can be amplified separately. After a 20-minute trial period without chloride addition, 0.04 ml of a molar sodium chloride solution is added to the electrolyte at five-minute intervals. The potential and potential noise, as well as the current noise between the steel electrodes, are measured and evaluated. Using the calculation of noise charge quantities and noise resistances, which were established in extensive preliminary research as characteristic values for the protective effect of pigments, an even finer differentiation of the results can be achieved, since the charge quantities show a direct relationship with the pigment effect.
Figure 4 shows the noise stream curves as a function of time over the entire 180-minute test period for non-alloy steel in aqueous binder with anticorrosive pigments P1 to P4.
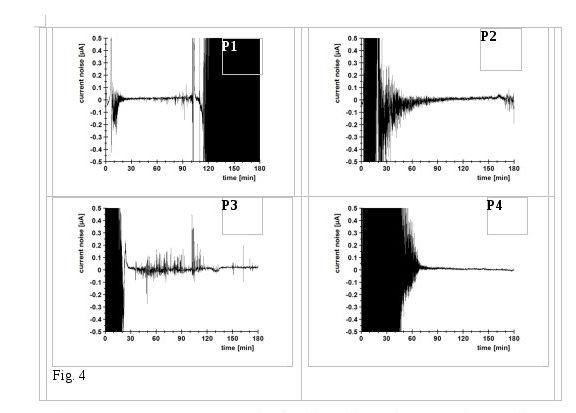
Figure 4: Current noise current curves as a function of time for the analysis of electrochemical noise in aqueous dispersions for coating
To illustrate the relationships and processes that take place on the metal surface, the cumulative charge amounts established from the noise current curves as a function of time are presented in Figure 5.
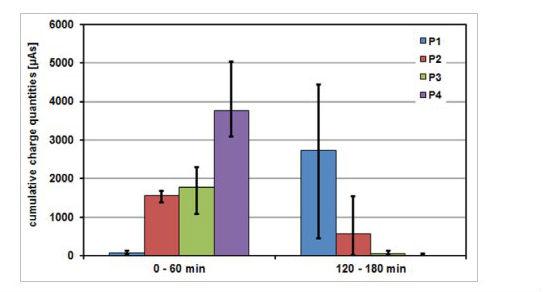
Figure 5: Cumulative charge quantities for two test intervals, established from the noise current curves as a function of time in the analysis of electrochemical noise in aqueous dispersions for coating.
Clear differences appear between the various pigments. While P1 at the start of the measurement has a low noise activity and therefore a low metal dissolution, a strong initial activity can be identified in the first test interval for the pigments P2, P3 and P4, due to the magnesium component. In the test period of 120 to 180 minutes, the noise activities of the pigment combinations P2 and P3 decrease greatly, while in the case of P1 there is an increase in dissolution. In fact, the P4 pigment shows a drastic drop in noise current and an amount of accumulated charge, however, the active dissolution of metal continues, which could be evidenced in the noise resistances detected.
Verification with conventional corrosion tests
To verify the evidence of electrochemical research methods using conventional corrosion tests, cold-rolled steel sheets were coated with a formulation used in practice, also based on an aqueous styrene acrylate with the anticorrosive pigments P1 to P4. Figure 6 presents the results, after 408 hours of aging in the salt spray test (DIN EN ISO 9227). The combination of the calcium and magnesium (P2) component in the anticorrosive pigment produces a significant increase in corrosion protection.

Figure 6: Test result after 408 hours of exposure to the salt spray test, base: aqueous styrene acrylate
To investigate the performance properties of the P2 pigment combination (CMP) in other binder systems, salt spray tests were performed with formulations based on a low-oil solvent-based alkyd resin (see Figure 7) and a solvent-based epoxy resin (see Figure 8).
To establish the comparison, the combination of pigments was tested against a zero sample without anticorrosive pigment, magnesium phosphate, calcium phosphate and a reference sample with zinc (zinc reference). The thickness of the dry coating in each case was 70 microns. To assess the degree of oxidation and oxidation creep in the cross-section, the lower half of the coating was always removed after the salt spray exposure test.
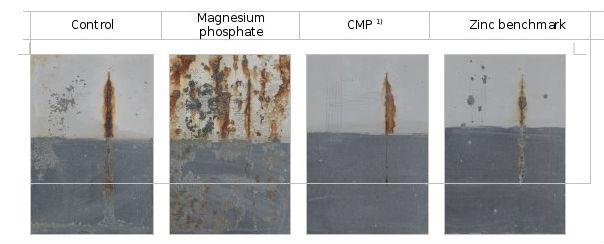
Figure 7: Test result after 408 hours of exposure in the salt spray test, base: alkyd resin with little solvent-based oil
The result of exposure to salt spray demonstrates good performance properties when using CMP, compared to magnesium phosphate. Even the protective effect of the reference sample containing zinc was outweighed with the use of CMP. Magnesium phosphate presents serious problems of adhesion, oxidation and blistering.
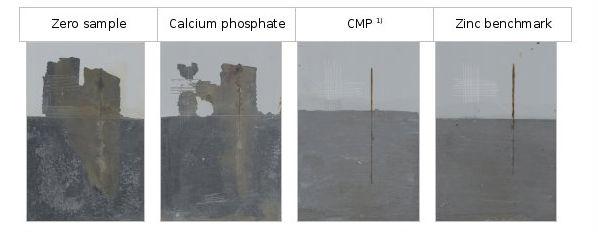
Figure 8: Test result after 504 hours of exposure in the salt spray test, base: solvent-based epoxy resin.
A significant improvement in oxidation adhesion and creep in the cross-section of this system could also be achieved by using CMP1).
Summary
It was possible to develop a new highly effective zinc-free pigment. Research using resting potential analysis and electrochemical noise analysis may show differences in pigment composition as well as its effect on substrate dissolution. The amount of the magnesium ratio in the pigment variants tested (P1-P4) has a decisive influence on the anticorrosive behavior of the system in general. Using resting potential analysis it can be clearly demonstrated that an increasing amount of the magnesium component in the anticorrosive pigment causes the value of the critical concentration to decrease, which provides an indication of the anticorrosive effect. A concentration of 100% of the magnesium component in the anticorrosive pigment causes the dissolution of active metal and does not provide protection against corrosion. Based on this research, it was possible to determine that P2 (CMP) could be an optimal pigment for this system, with a defined Ca/Mg ratio.
With respect to electrochemical noise, the tendency is first identified that the initial noise activity in the time interval of 0 to 60 minutes increases with increasing concentration of the magnesium component. This behavior points towards a greater dissolution of the substrate in the first 60 minutes of the measurement by increasing the concentration of magnesium. After a prolonged testing period and the addition of chloride, the P2 pigment combination showed the best anticorrosive properties. P4 pigment with 100% magnesium component, in fact, shows a reduction in noise activity. By including the established noise resistances, it can be shown, however, that further dissolution of the metal continues to take place.
Results
The identification and use of synergistic interactions is useful in the development of new, high-efficacy anticorrosive pigments.
The results of electrochemical investigations supported the choice of suitable synergistic components, which considerably reduces the time required to carry out extensive preliminary investigations.
Modern electrochemical research methods such as resting potential analysis and ECN were successfully used.
It was possible to confirm the good result of the new pigment CMP1) in electrochemical investigations by conventional corrosion tests.
Note: 1. Pigments used: CMP is Heucophos® CMP (magnesium and calcium orthophosphate) produced by Heubach GmbH, Langelsheim, Germany.
References
[6] Heyn, A., Göllner, J.: "Analysis and Monitoring of Corrosion using Electrochemical Noise - 5(th) Part", Materials and Corrosion (Vol. 64), No. 8, 2013, p. 663
[7] Plagemann, P.; Yezerska, O.; Brinkmann, A.: [2] L. Kirmaier, Farbe und Lack, (2009), 115, p. 94-97
* Lars Kirmaier was born in Berlin in 1970. He studied chemistry and completed his doctoral thesis on highly reactive silicon intermediates in 1998. He joined the R&D department of a leading German painting company and gained practical experience in solvent-free and high-solids epoxy and polyurethane resin applications. He started with Heubach GmbH in January 2004 as laboratory manager for anticorrosive pigments and joined the technical marketing team in January 2012. Learn more at www.heubachcolor.com























Leave your comment