 In this article the author studied the behavior that exposes the basic aluminum benzoate as a pigment that inhibits corrosion of steel and its application in anticorrosive paints.
In this article the author studied the behavior that exposes the basic aluminum benzoate as a pigment that inhibits corrosion of steel and its application in anticorrosive paints.
by Dr. Guillermo Blustein*
Its inhibitory action, in aqueous suspension, was determined by electrochemical tests (corrosion potential and linear polarization tests) and surface analysis techniques (SEM and EDAX), on SAE 1010 steel electrodes. After the anticorrosive efficiency of this product was verified, anticorrosive paints were formulated and applied on steel panels.
Protective efficiency was assessed by accelerated aging (salt spray chamber) and electrochemical (electrochemical impedance spectroscopy) assays.
The results obtained indicate that paints formulated with this new pigment exhibit a similar or superior anticorrosive capacity than those formulated with classic non-toxic pigments (example: zinc phosphate), especially in water-based paints; which profiles it as an excellent substitute for the anticorrosive pigments used to date.
Initial analysis
The anticorrosive paints traditionally used that contain pigments based on chromium and lead compounds have been questioned in developed countries for their high danger to health and pollution of the environment. There is currently a global trend to remove these pigments and replace them with others with low environmental impact [1].
In the search for new anticorrosive pigments, friendly to the environment and with good corrosion inhibitory properties, it was thought to study metal benzoates. This choice was not arbitrary but was based on the hypothesis of being able to take advantage of the known inhibitory properties of corrosion that the benzoate anion has in an aqueous medium.
The properties of the benzoate anion to inhibit iron corrosion are well known based on studies carried out in a timely manner and which used sodium benzoate and benzoic acid in different media, including those containing the chloride anion [2-16].
The use of benzoate in mixtures with other inhibitory anions such as gluconate and acetate has also been reported [17] and, more recently, the possibility of also using calcium benzoate in neutral media [18]. In addition to studies on steel, sodium benzoate and benzoic acid have been successfully used on other metals such as aluminum [19-23], zinc [24, 25], copper [26] and bismuth [27].
The electro-adsorption mechanism of benzoate has been studied on gold electrodes [28, 29] and that of benzoic acid on platinum [30], copper [31] and silver electrodes [32]. These studies contributed much to the mechanism proposed for steel with the same inhibitors, which begins with the adsorption of the inhibitor on the active sites of the metal surface, producing an effective covering of the same and, therefore, a notable decrease in the rate of corrosion [33, 34].
The use of ammonium benzoate as an inhibitor of gas-phase iron corrosion [35] has also been reported, usually in small instruments packed for transport.
Soluble salts of benzoic acid were also used in the field of concrete technology as oxidation inhibitor additives for rebar [36, 37], and in the field of organic coatings. In the latter, the applications have been diverse and among them could be cited its use in additives that inhibit the so-called "flash rusting" that occurs during the painting of steel with water-based paints [38-45], in biologically active compounds for antifouling paints [46-49], etc.
The use of benzoic acid and sodium benzoate as inhibitory pigments in anticorrosive paints is not possible since their high solubility would produce a rapid leaching from the film, leaving it quickly without inhibitor reserve and excessively increasing its permeability, with the consequent loss of its protective properties. On the other hand, excessively insoluble compounds would not provide enough anion for adequate protection. In this sense, the present work was aimed at achieving the synthesis of metal benzoates, in particular the basic aluminum benzoate to satisfy the aforementioned restrictions and thus be incorporated into the formulation of an anticorrosive cover.
It is worth mentioning that the choice of this anion attends not only to the above but also to economic reasons (it is a low-cost industrial product) and ecological reasons (benzoic acid and sodium benzoate are widely used as additives for the preservation of food for human consumption).
Experimental part
- Preparation and characterization of aluminum benzoate: This compound was prepared in the laboratory by precipitation from ammonium benzoate and ferric nitrate, adjusting to a final pH of 3.5 [50]. The pigment was characterized with regard to its composition and pH of the aqueous extract.
At the same time, the corrosion potential was determined as a function of time for SAE 1010 steel specimens immersed in a suspension of the inhibitor in NaClO4 0.025 M, with and without zinc oxide. The polarization curves were obtained in electrolytes formed by aluminum benzoate suspensions with and without zinc oxide in NaClO4 0.5M. A conventional three-electrode cell with a large area platinum counterelectrode and a saturated calomel electrode was used as a reference. SaE 1010 steel included in Teflon was used as a working electrode, with an exposed area of 0.20cm2 and polished with 600 emery. The sweep speed was 0.003V.s-1 in all cases.
Finally, the morphology and composition of the protective films formed on the steel after immersion in the suspensions of the inhibitory pigment, in the presence and absence of zinc oxide, was studied by means of scanning electron microscopy (SEM) coupled with the EDAX probe.
- Formulation, elaboration and application of the paints: The paints were formulated with an anticorrosive pigment content of 30% by volume on the total pigments. Titanium dioxide, talc and barite were used as complementary pigments; binders, an alkyd resin and an epoxy/polyamide (solvent-based paints) and a polyamine-amide epoxy resin (water-based paint); mineral turpentine solvents for alkyd paints, xylene/methyl isobutyl ketone/butyl acetate for solvent-based epoxy and water for water-based epoxy. The solvent-based paints were prepared in a 1l capacity ball mill and the water-based paint in a high-speed disperser. The paints were applied with a brush up to a final dry film thickness of 70+_5m, on SAE 1010 steel specimens previously sandblasted and degreased with toluene.
- Tests on painted panels: The protective efficiency of the paints was periodically evaluated through the degree of oxidation (ASTM D 610) presented by the painted panels exposed in the salt spray chamber (ASTM D 117).
The protection provided by the anticorrosive pigment was monitored through electrochemical impedance measurements, which were carried out in a conventional three-electrode cell. The painted specimen was the working electrode, a Pt-Rh mesh of negligible impedance the counter electrode and a saturated calomel electrode (ECS) the reference. The impedance spectra as a function of immersion time in a solution of NaClO4 0.5 M and NaCl 0.5 M were performed in the potentiostatic mode, at the corrosion potential, in the frequency range 5.10-3Hz<f<1.106Hz; the signal amplitude was 10mV. The processing of the data was carried out by a set of programs developed by Boukamp [51].
Results and discussion
- Chemical characterization: The stoichiometric relationship between the Al+3 cation and the benzoate anion suggests that the minimum formula of the compound formed is Al(C7H5O2)2OH [50].
The pH of the aluminum benzoate suspension (~3.58) shows that it alone is not suitable for formulating a paint since the protection of the steel starts at pH 7. Therefore, zinc oxide was added to raise the pH (~6.86) and thus lead to the formation of an efficient protective film. In addition, the Zn++ cation polarizes cathodic areas by precipitation of zinc oxyhydroxides.
- Electrochemical characterization: Due to the high hydrolytic acidity of aluminum benzoate, the corrosion potential of steel in a suspension of the same presents typical values of steel in frank dissolution (~ -0.50V vs. ECS). The incorporation of ZnO produced a notable displacement of the corrosion potential towards nobler values (~ -0.20V vs. ECS), that is, towards the passivity zone. This shift is greater than when the corrosion potential is measured only in the presence of zinc oxide (~-0.35V vs. ECS) which demonstrates clear synergism between both pigments, see Fig. 1.
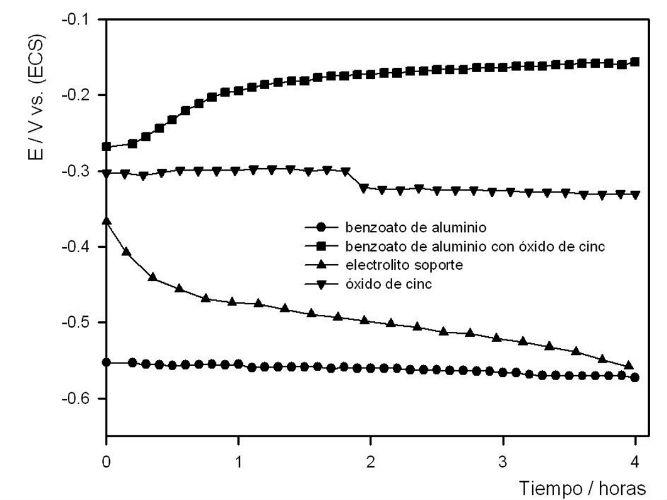
Fig. 1. Corrosion potential of steel.
Tafel polarization curves show that in the absence of zinc oxide, the corrosion current is high and the steel in this medium is in frank dissolution, Fig. 2. Again, the incorporation of zinc oxide markedly decreased the corrosion current as clearly seen in Figure 2.
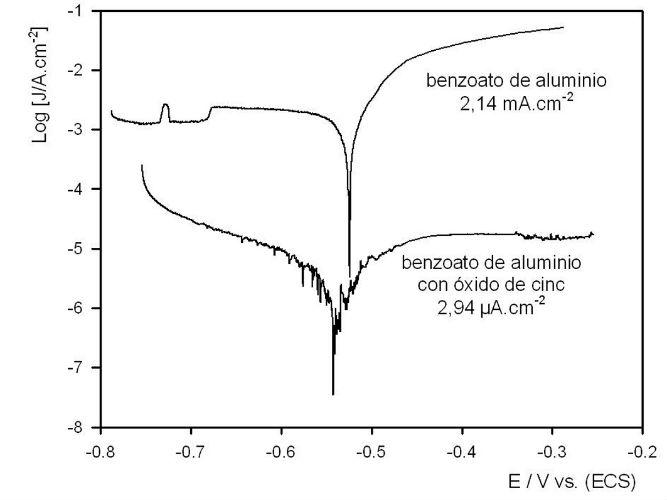
Fig. 2. Polarization curves.
Note: This article represents a part of dr. Guillermo Blustein's doctoral thesis work presented at the Faculty of Exact Sciences-National University of La Plata.
* Dr. Guillermo Blustein* CIDEPINT- Centro de Investigación y Desarrollo en Tecnología de Pinturas Calle 52 e/ 121 y 122 (B1900AYB) La Plata. Argentina. [email protected]
References
1. S. Krieg. Lead and chromate free anticorrosive pigments based on phosphates. Pitt. & Vern., 1996, 72 (12), 18.
2. Mr. Eurof Davies and Q. J.M. Slaiman. Mechanism of the corrosion inhibition of Fe by sodium benzoate – I. The influence of concentration and pH in air-saturated solutions of sodium benzoate. Corr. Sci. 11 1971 p 671.
3. Q. J.M. Slaiman and D. Eurof Davies. Mechanism of the corrosion inhibition of Fe by sodium benzoate –II. The inhibitive properties of sodium benzoate in de-aerated and air-saturated solution. Corr. Sci. 11 1971 p. 683.
4. Mr. Eurof Davies and Q. J.M. Slaiman. Mechanism of the corrosion inhibition of iron by sodium benzoate – III. The role of oxygen. Corr. Sci. 13 (11)1973 p. 891.
5. V.S. Muralidharan, R. Sethuraman, S.Krishnamoorthy. Benzoic acid as corrosion inhibitors for pure iron in sulphuric acid. Bull. Electrochem. 4 1988 p 705.
6. D. S. Azambuja, L. R. Holzle, I. L. Muller and C.M. S. Piatnicki. Electrochemical behaviour of iron in neutral solutions of acetate and benzoate anions. Corr. Sci. 41 (11) 1999 p. 2083.
7. Masahiko Yamaguchi, Hiroshi Nishihara and Kunitsugu Aramaki. The inhibition of passive film breakdown on iron in a borate buffer solution containing chloride ions by anion inhibitors. Corr. Sci. 36 (2) 1994 p. 241.
8. Mübeccel Ergun and Ya Sar Turan. Pitting potential and protection potential of carbon steel for chloride ion and the effectiveness of different inhibiting anions. Corr. Sci. 32 (10) 1991 p. 1137.
9. V. Otieno-Alego, G. A. Hope, H. J. Flitt, G. A. Cash and D. P. Schweinsberg. The effect of potential scan on the parameters used to synthesize anodic polarization curves. Corr. Sci. 33 (11) 1992 p. 1719.
10. R. Kahraman, A.A. Al-Mathami, H. Saricimen, N. Abbas and S.U. Arman. A study of corrosion control of carbon steel using inhibitors in a simulated enviroment. Anti-Corrosion Methods and Materials 49 (5) 2002 p. 346.
11. P. Argawal and D. Landolt. Effect of anions on the efficiency of aromatic carboxylic acid corrosion inhibitors in near neutral media: Experimental investigation and theoretical modeling. Corr. Sci. (4/5) 1998 p. 673.
12. Kahraman R. Inhibition of atmospheric corrosion of mild steel by sodium benzoate treatment. J. Mat. Eng. and Performance 11 (1) 2002 p. 46.
13. K. Takahashi, J. A. Bardwell, B. Mac Dougall and M. J. Graham. Mechanism of anodic dissolution and passivation of iron--II. Comparison of the behavior in neutral benzoate and acetate buffer solutions. Electrochimica Acta 37 (3) 1992 p. 489.
14. J. R. Culleré and M. Lluveras. Mechanism of inhibitory action of sodium benzoate. Rev. Iberoam. Corr. Prot. 1983 p. 225.
15. Gianluca Bondietti, Jürg Sinniger and Werner Stumm. The reactivity of Fe (III) (hydr)oxides: Effects of ligands in inhibiting the dissolution. Colloids and Surfaces A: Physicochemical and Engineering Aspect 79 (2-3) 1993 p. 157.
16. R. Kahraman, H. Saricimen, Al-Zahrani and S. Al-Dulaijan. Effect of inhibitor treatment on corrosion of steel in a salt solution. J. Mat. Eng. Performance 12 (5) 2003 p. 524.
17. O. Lahodny-Šarc and F. Kapor. Corrosion inhibition of carbon steel by blends of gluconate/benzoate at temperatures up to 60ºC. Mat. Sci. Forum 289-292 1998 p. 1205.
18. G. Blustein, J. Rodríguez, R. Romagnoli and C. F. Zinola. Inhibition of steel corrosion by calcium benzoate adsorption in nitrate solutions. Corr. Sci. 47 (2005) 369 –383
19. P. N. S. Yadav, A. K. Singh and R. Wadhwani. Role of hydroxyl group in the inhibitive action of benzoic acid towards corrosion of aluminium in nitric acid. Corrosion (NACE) 55 (10)1999 p. 937.
20. Raspini. Influence of sodium salts of organic acid as additives on localized corrosion of aluminium and its alloys. Corrosion (NACE) 49 (10)1993 p. 821.
21. A.K. Mohamed, S.A. Abd El-Maksoud and A.S. Fonda. p-Substituted benzoic acid derivatives as corrosion inhibitors for aluminium in H3PO4. Portugaliae Electrochimica Acta 15 1997 p. 27.
22. Sibel Zor. The effects of benzoic acid in chloride solutions on the corrosion of iron and aluminium. Turk J. Chem. 26 2002 p. 403.
23. W. J. Rudd and Scully. The function of the repassivation process in the inhibition of pitting corrosion on aluminium. Corr. Sci. 20 (5)1980 p. 611.
24. Kanitsugu Aramaki. Effects of organic inhibitors on corrosion of zinc in aerated 0.5 M NaCl solution. Corr. Sci. 43 (10) 2001 p. 1985.
25. S.M. Abd El Haleem and A. A. Abdel Fattah. The role of some organic anions in promoting or inhibiting the corrosion of zinc. Surf. Coat. Technol. 29 (1) 1986 p. 41.
26. S. N. Mostafa, M. Y. Mourand and S. A. Seliman. Electrochemical studies on the corrosion of cooper in organic electrolyte solutions. J. Electroanal. Chem. 130-1981 p. 221.
27. A. Ammar and M. W. Khalil. Behaviour of bismuth as valve metal in phosphate, borate, benzoate and tartrate solutions. J. Electroanal. Chem.32 (3) 1971 p. 373.
28. Hong-Qiang Li, Sharon G. Roscoe and Jacet Lipkowski. FTIR studies of benzoate adsorption on the Au (111) electrode. J. Electroanal. Chem.478 (1-2) 1999 p. 67.
29. P. Zelenay, P. Waszczuk, K. Dobrowolska and J. Sobkowski. Adsorption of benzoic acid on a polycrystalline gold electrode. Electrochimica Acta 39 (5) 1994 p. 655.
30. P. Zelenay, and J. Sobkowski. Electrosorption of benzoic acid on platinum electrode. Electrochimica Acta 29 (12) 1984 p. 1715.
31. P. Waszczuk, P. Zelenay, and J. Sobkowski. Surface interaction of benzoic acid with a cooper electrode. Electrochimica Acta 40 (11) 1995 p. 1717.
32. P. Waszczuk, P. Zelenay and J. Sobkowski. Radiometric and voltammetric study of benzoic acid adsorption on a polycrystalline silver electrode. Electrochimica Acta 43 (14-15) 1998 p. 1963.
33. G. Blustein and C. F. Zinola. Inhibition of steel corrosion by calcium benzoate adsorption in nitrate solutions: theoretical and experimental approach. J. Colloid and Interface Sci. 278 (2004) 393-403
34. P. Kern and D. Landolt. Adsorption of organic corrosion inhibitors on iron in the active and passive state. A replacement reaction between inhibitor and water studied with the rotating quartz crystal microbalance. Electrochimica Acta 47 (4) 2001 p. 589.
35. A. Leng and Stratmann. The inhibition of the atmospheric corrosion of iron by vapour-phase-inhibitors. Corr. Sci. 34 (10) 1993 p. 1657.
36. Monticelli, A. Frignani and G. Trabanelli. A study on corrosion inhibitors for concrete application. Cement and Concrete Researcher 30 2000 p. 635.
37. James M. Gaidis. Chemistry of corrosion inhibitors. Cement & Concrete Composites 26 2004 p. 181.
38. F. Galliano and D. Landolt. Evaluation of corrosion protection properties of additives for waterborne epoxy coatings on steel. Prog. Org. Coat. 44 (3) 2002 p. 217.
39. S. A Hodges, W.M. Uphues and M. T. Tran. Non-toxic corrosion inhibitive synergistic system. Surf. Coat. Intern.4 1997 p. 178.
40.C.H. Simpson. US Navy Develops Non-toxic, self-priming coatings for aluminium and steel. Paint & Coatings Industry 1993.
41.M.A. Jackson. Formulation of waterborne epoxy primers: An evaluation of anticorrosive pigment. J. Protective Coating and Lining 1990.
42. Andréa Kalendová. Methods for testing and evaluating the flash corrosion. Prog. Org. Coat. 44 (3) 2002 p.201.
43. Halox® Flash-X330 Halox Flash Rust Inhibitors.
44. Gail Pollano and Amy Lurier (Polyvinyl Chemicals Inc.). Factors affecting salt spray resistance of an aqueous coating on metal. Paint & Coatings Industry May/June 1987.
45. S. Gee, Surfing. Coat. Intern. 80 (1997) 316.
46. I. Chet, P. Asketh, and R. Mitchell. Repulsion of bacteria from marine surfaces. Applied Microbiology. 30-1975 p. 1043.
47. Mirta Stupak, Mónica García, Miriam C. Pérez. Non-toxic alternative compounds for marine antifouling paints. International Biodeterioration & Biodegradation 52 2003 p. 49.
48. V. Vetere, M. Pérez, M. García, M. Deyá, M. Stupak and B. del Amo. A non-toxic antifouling compound for marine paints. Surf. Coat. Intern. 12 1999 p. 586.
49.M. Pérez, M. García, V. Vetere, M. Deyá, B. del Amo and M. Stupak. Benzoates: a new approach to non-toxic marine fouling control. Pigment & Resin Technology 30 (1) 2001 p. 34.
50. Blustein, G., "Desarrollo de inhibidores a base de benzoatos metálicos para la protección anticorrosiva del acero", Doctoral Thesis, Dept. of Chemistry, Universidad Nacional de La Plata, La Plata, Argentina (2005).
51. Boukamp, B.A., Report CT88/265/128, CT89/214/128, Equivalent Circuit, University of Twente, The Netherlands (1989).
52.M.C. Deyá, G. Blustein, R. Romagnoli and B. del Amo. The influence of the anion type on the anticorrosive behaviour of inorganic phosphates. Surf. Coat. Technol. 150 (2-3), 2002 133-142.
53. G. Blustein, B. del Amo and R. Romagnoli. The influence of the solubility of zinc phosphate pigments on their anticorrosive behaviour. Pig. & Res. Technol. 29(2) 2000 100-107.ence: 41, 2083-2097 (1999).























Leave your comment