 In the following article we expose the identification of specific synergies using electrochemical corrosion investigations.
In the following article we expose the identification of specific synergies using electrochemical corrosion investigations.
by Dr. Lars Kirmaier*
The development of anticorrosive pigments for the most varied coating systems is an extremely slow and expensive process, due to the climatic tests that must be performed, such as salt spray exposure tests.
To accelerate work towards the development of a new zinc-free pigment, offering better protection, modern electrochemical research methods have been successfully employed, and verified through the use of conventional tests [1].
Mechanism of action of conventional anticorrosive phosphate pigments: the use of suitable anticorrosive pigments significantly affects the protective properties of formulations for metal substrates. The mechanism of action of an anticorrosive pigment is attributed to the following factors [2, 3]:
- Increased film resistance
- Prevention of oxidation creep and corrosion below the film in defective areas
- General delay of the corrosion process
- Cathodic and/or anodic passivation of the metal surface
In the case of zinc phosphates, a very low solubility in water causes the release of secondary phosphate ions into the coating, which are responsible for the formation of inhibitory adhesive complexes on the metal surface and the associated anodic passivation.
Another theory on the mechanism of action also describes the formation of tribasic ferrous phosphate complexes, although only in slightly acidic media [4, 5]. As an ampholyte, zinc, or zinc hydroxide resulting from hydrolysis, also exhibits a solubility behavior in acidic and alkaline media that is advantageous for corrosion protection.
The zinc orthophosphate pigments and modified zinc polyphosphate pigments that are already established in the market, show a substantial increase in chemical and electrochemical effectiveness, compared to traditional zinc phosphate, which allows to achieve very good protection properties. In addition to aluminum, molybdate and organically modified types (ZPA, ZMP, ZPO), the universal WSA pigments ZCP PLUS and ZAM PLUS stand out.
In addition to economic considerations, ecological and regulatory factors are now playing an increasingly decisive role in the formulation of innovative coating systems. It is therefore not surprising that the tendency to develop anticorrosive pigments free of zinc or pigments that do not require labeling has increased continuously in recent years.
Zinc-free technology is not new, numerous pigments based on calcium, strontium, aluminum and magnesium phosphate have been available on the market for a long time. However, the real drawback is that only in very few cases is it possible to combine a universal application with very good corrosion protection, as is the case with modified zinc phosphates. Although there are other causes, this is mainly due to the difference in solubility characteristics, compared to zinc phosphate, of the corresponding compounds.
Although the periodic table offers several alternatives to zinc that do not contain heavy metals, only a few metals meet the characteristics of suitable contractions. When making a selection, emphasis is therefore placed on the possible positive interactions between calcium phosphate compounds and magnesium. The following were the requirements set for the new zinc-free pigment:
- Zinc-free pigment technology
- Highly effective anodic corrosion protection in solvent- and water-based systems
- Stability and universal application
- Very good dispersion properties
- Economy
Even in the early stage of the investigations, exceptional increases in performance were observed when using the newly developed pigments with a different magnesium-calcium ratio, which also had a positive effect on salt spray resistance.
Electrochemical investigations directly in dispersions for coating: after exhaustive preliminary investigations the test conditions for two electrochemical methods could be determined. Firstly, the analysis of the resting potential and, secondly, the analysis of electrochemical noise, which allow comparisons to be made on the protective effect of anticorrosive pigments, dispersed in an aqueous binder.
In all electrochemical investigations, a round bar of non-alloy steel (C55, material No.: 1.1203) was used as a sensor or working electrode. As an electrolyte, a dispersion for aqueous, organic coating was used, which was produced by dispersing the binder and the respective anticorrosive pigment by means of a solvent. Because the organic coating dispersions were, in fact, aqueous, but highly viscous, they were diluted in a 50:50 ratio with deionized water.
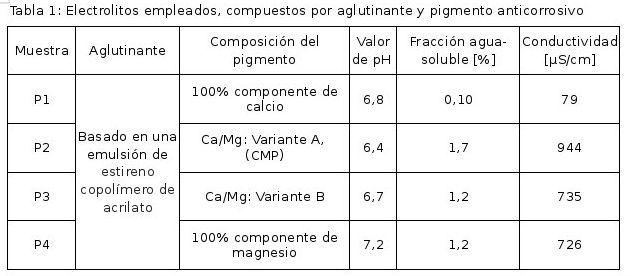
Table 1 shows the electrolytes used, composed of an aqueous binder and four different anticorrosive pigments or combinations of pigments with a different ratio of Ca/Mg. Variant B shows a higher magnesium content than variant A. It is observed that the pigment P2 (CMP) exhibits the lowest pH value at the highest solubility and conductivity. This makes it difficult to predict the actual efficacy of this combination of pigments in real coating systems. However, it is a first indication of modified electrochemical behavior.
Electrochemical analysis of the resting potential
Rest potential analysis (RPA) is based on a measurement of the resting potential using a two-electrode configuration, for which an Ag/AgCl electrode was used as the reference electrode and C55 non-alloy steel as the working electrode. As an electrolyte, dispersion is used for aqueous coating (see Table 1), which is stirred throughout the measurement with a magnetic stirrer to prevent pigments from settling. At defined time intervals, a solution of molar sodium chloride (as a corrosion stimulator) is added to the electrolyte via a computerized pump.
As a fundamental measurement parameter, the potential curve is observed and evaluated by determining a critical amount of chloride in which a significant potential drop occurs. For all measurements, the potential curve is first recorded for 60 minutes without the addition of chloride.
As a characteristic example of the resting potential analysis, Figure 2 shows a resting potential curve for each anticorrosive pigment, P1 to P4. Using the potential curves, differences in the behavior of the pigments can be observed. From P1 to P3, the marked drop in potential can be understood as a result of the addition of a defined amount of chloride. For P4 it is observed that the resting potential arises right at the beginning of the investigation at -400 mV.
In this potential there is a strong dissolution of the metal that accelerates further after 60 min. as a result of the addition of chloride. The slight increase in potential to approximately -250 mV is attributed to corrosion or by-products on the metal surface and not to inhibitory mechanisms.
Pigments P2 (CMP) and P3 are combinations of pigments P1 and P4 in different proportions. Pigment combinations first present the characteristic initial potential drop to approximately -400 mV and then an increase in potential as a function of the amount of P4 pigment added. The greater the amount of P4 pigment (100% magnesium component) that is added to the pigment combination, the more time the metal requires to develop a passive-like surface state and therefore the longer it takes for the potential increase to occur.
Figure 3 shows the critical amounts of chloride identified in the potential drop that characterizes the anticorrosive pigments P1 to P4, dispersed in the binder. In each case the mean value is displayed, calculated using a minimum of three individual measurements. Pigment P2 (CMP) exhibits the highest critical concentration value, while pigment P1 and especially P4 exhibit the lowest values.
Note: The figures in this article can be seen in the print or digital edition.
References
[1] S. Bender, M. Babutzka, L. Kirmaier: "Moderne elektrochemische Korrosionsuntersuchungen gezielt eingesetzt", Farbe und Lack, (2014), in press
[2] L. Kirmaier, Farbe und Lack, (2009), 115, p. 120-123
[3] Vogelsang, J., Basics about Anticorrosive pigments and Corrosion Inhibitors and Possibilities for their Usage, European Coatings Conference, Berlin, 2000
[4] Ruf, J., Organischer Metallschutz, Vincentz Verlag, Hannover, 1993, 260
[5] Yongsheng Hao, Fuchun Liu, En-Hou Han, Saima Anjum, Guobao Xu, Corrosion Science, 2013, 69, p. 77-86
* Lars Kirmaier was born in Berlin in 1970. He studied chemistry and completed his doctoral thesis on highly reactive silicon intermediates in 1998. He joined the R&D department of a leading German painting company and gained practical experience in solvent-free and high-solids epoxy and polyurethane resin applications. He started with Heubach GmbH in January 2004 as laboratory manager for anticorrosive pigments and joined the technical marketing team in January 2012. Learn more at www.heubachcolor.com























Leave your comment