 A chemical engineer immersed in all phases of the paints and coatings industry must be clear about each of the technical concepts that are handled. Unifying them will allow us to better understand the language of our sector.
A chemical engineer immersed in all phases of the paints and coatings industry must be clear about each of the technical concepts that are handled. Unifying them will allow us to better understand the language of our sector.
by Jordi Calvo Carbonell
Often the technical terminology comes from the popular lexicon, at the beginning of the industrial manufacture of paints, during the last quarter of the nineteenth century and the first of the twentieth, the existing vocabulary was accommodated to define certain characteristics of these. The word drying, whose meaning was the total evaporation of the water contained in a certain object was used to define the time necessary for a painting to completely lose stickiness, because the paint was a liquid product and the loss of stickiness constituted the reliable proof of the total evaporation of volatile elements.
Today the words dried and cured are used in a somewhat imprecise way and sometimes without following specific rules, this is a pending issue that in some cases must be corrected.
With this prologue we only wanted to introduce the reader to the lack of concreteness that occurs in many cases in technical terminology, however there is still a wider distance between the vocabulary used by manufacturers and users that considerably compromises the interpretation that the latter make about what is stated in the technical sheets of each product.
Drying versus curing
We now begin the comparison between the terms drying and curing. In the first place it is necessary to separate the liquid paints into two groups, the first is the one in which the total evaporation of volatile elements produces a film whose characteristics are definitive, the second group is formed by the paints in which after the evaporation of the volatile substances a chemical reaction must still be produced or finished that will entail a progressive change of the characteristics until obtaining the definitive ones.
The first group is formed by those products whose formation process is limited to the evaporation of solvents, that is, what we know as "physical drying", such is the case of dispersion polymers, nitrocellulosic paints, etc. These products once formed the film composed of a 100% solid material has characteristics that can be considered definitive.
In the second group it is formed by products into one or two components that are transformed due to a chemical reaction, these products once evaporated the solvent are subjected to changes that occur while the reaction persists and to reach the definitive characteristics they require time, application of heat, radiation, etc.
Such is the case of paints based on oil or alkyd resins that require a certain time for the oxidation reaction to occur on the double bonds. Two-component products based on epoxy resins or polyurethane paints once they lose their stickiness have characteristics that are not yet definitive and that vary over time, nor can we forget the bakable paints based on combinations of certain resins with amine resins in which the evaporation of solvents is only a first step of initiation of the reaction that is completed by the application of heat.
Drying
As already indicated, drying occurs from the moment the volatile components of the paint have evaporated. In general, two cases can occur: physical drying and oxidation drying, which in fact should be considered within the group of products that cure, but historically the word drying has been used to define its process to obtain the final characteristics.
- Physical drying: a paint has a physical drying process when this is produced by simple evaporation of solvents, once these have evaporated there are no chemical changes that modify their characteristics. Within this group can be found paints based on polymers in dispersion (plastic paints), paints based on nitrocellulose, chlorinated rubber or cycled rubber, etc.
In Figure 1, you can see the variation of the pendulum hardness in a paint whose drying is produced by the simple evaporation of the solvents, the hardness curve reaches practically its maximum in a few hours and the slight increase in this is given by the elimination of the heavier solvents that can sometimes occur in a longer time. In the case of dispersion polymers, for example, containing coalescing, the final hardness is not obtained until several days or weeks after application, in fact the resistance to rubbing tests in plastic paints are standardized to be carried out 21 days after application.
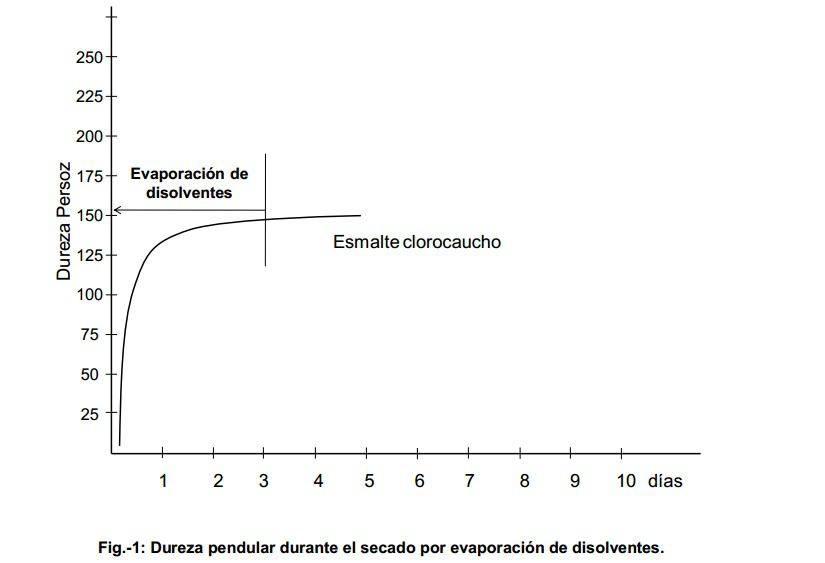
- Oxidation drying: when once the solvents have evaporated, the paint requires a process of reaction of the oxygen from the air with the double bonds of the oils in order to lose the stickiness and obtain the final hardness, it is said that its drying is produced by oxidation.
Such is the case of paints based on oils or alkyd resins whose hardening process is delayed several hours or even days after the evaporation of solvents. This is an example of uncertainty in technical vocabulary.
Cured
In general, the term curing is used to define the process of obtaining the final characteristics, drying, in paints that are presented in two components or that require a chemical reaction to achieve this objective.
In this section we can cite by way of example polyurethane paints formed by a base component that has a certain content in hydroxyl groups and a hardener based on an isocyanate or epoxy paints whose pigmented component is generally based on an epoxy resin and the hardener based on an amine, polyamide, etc.
In general, paints that require a chemical reaction between two components or free radicals, carbonation, environmental humidity, etc. are said to cure or have a certain curing time.
Pot-life
When the two components of a paint are mixed, the reaction of the base component and the hardener begins, if this time exceeds a certain time the reaction has advanced enough so that the resulting film does not have the characteristics for which it has been designed, such is the case of wash-primers, liquid epoxy paints and polyurethane paints.
The shelf life or Pot-life is the time during which a two-component paint once mixed, maintains its characteristics and can be applied with guarantee that the characteristics of the resulting film are the same as those obtained with a freshly prepared mixture.
Pot-life considerations
We know that the reaction rate of two products is constant as long as the conditions under which the reaction is performed remain so. This statement leads us to verify whether in all cases the conditions remain constant. It is therefore necessary to take into account that if the reaction is exothermic the internal area of the mass of the mixture will be in contact with a product whose temperature increases as the reaction progresses while the external zone will be in contact with the air whose temperature will remain constant.
The above leads us to justify that the greater the volume of the mixture, the faster the reaction speed will be since the reaction heat itself increases the temperature and therefore reduces the reaction time or the pot-life. A classic example can be the mixture of a liquid epoxy resin with a cycloaliphatic amine, both products 100% solid matter. In order to clarify this point we can consider a mixture of a volume of 200 cc. of both products whose hardening occurs in a time of 20 to 30 minutes while a film of 500 wet microns of the same sample can maintain stickiness for several hours.
Another section is paints with a high content of solvents, whether organic or water, where the possible exothermia is practically zero and the physical changes in the paint mixture are at first glance negligible except for a slight change in viscosity due to the evaporation of the solvents. The advance of the reaction in this case entails the formation of long and crisscrossed polymer chains with solvent entrapment that when applied are destroyed and are not able to form a continuous and homogeneous film. A wash-primer and an epoxy paint or a polyurethane paint can serve as an example, in all of them problems of lack of adhesion may appear and in the last two, loss of shine, exudations and / or deficient chemical resistance.
Determination of the pot-life
When it comes to 100% solid paints, with an exothermic reaction, the potlife is defined by the time during which the paint can be applied and taking into account the speed of the reaction and the solidification of the same its determination is made by simulation by executing a mixture of determined volume, usually 200 cc, and the determination of solidification time.
The question therefore arises, what is the use of the determination of the pot-life in volumes of 200 cc if the application is carried out with mixtures of much higher volumes? The answer is twofold, firstly it is a quality control parameter that ensures that the two components react correctly, secondly it indicates to the applicator that it must apply the product under appropriate conditions: Application of the entire mixture in the shortest possible time in order to expand the surface and therefore facilitate the dissipation of the heat of reaction or apply the product with a gun of mixing in nozzle so that the contact time of the two components before application practically zero.
The pot-life should never be confused with the curing time since in the application of the paint the thicknesses can be from 500 microns to several millimeters and the spectacular increase in temperature that occurs en masse does not occur in the film.
In paints whose solvent content is sufficient so that there are no appreciable visual changes, there is a danger that once the mixture has been made, enough time will pass so that the reaction in the packaging is so advanced that the resulting film has defects or does not provide the characteristics for which it has been designed. As already indicated, we have examples of this in the wash-primer of two components that must have good adhesion on certain metals such as zinc, aluminum or light metal alloys, if the product is applied after several hours of its mixture it can be observed that the adhesion obtained is insufficient. In the case of epoxy paints, a lack of hardness or even oily exudations on the surface can be observed.
Note: In the next edition we will publish the second part of this article, which will talk about the quantification of drying and curing times.
* Jordi Calvo is a chemical engineer, member of the Board of Directors of AETEPA (Spanish Association of Paint and Allied Technicians) and author of the book "Paintings and Coatings: Introduction to their technology".























Leave your comment