 Below we analyze the way in which natural iron oxides act as "barrier" pigments in the formulation of anticorrosive paints.
Below we analyze the way in which natural iron oxides act as "barrier" pigments in the formulation of anticorrosive paints.
by Luciano Cuesta Orcoyen*
Corrosion is one of the most serious problems facing modern societies. The annual costs related to corrosion and corrosion prevention have been estimated to constitute a significant part of the gross national product in several countries of the developed world [1]. In addition to the economic costs and technological delays they cause, corrosion can lead to structural failures that can have dramatic consequences for humans [2]. Although many techniques have been developed to reduce or minimize the action of corrosion, the use of anticorrosive paints or coatings is the most efficient, economical and most widely used system today for this purpose.
Anticorrosive paints
An anticorrosive coating system usually consists of multiple layers of paint with different properties and effects (see Figure 1).3 In an anticorrosive system typical for highly corrosive environments (industrial zones, marine environments, etc.) we usually find three zones, an initial layer called "primer" or primer layer, one or several intermediate layers, and a finishing layer.
The essential functions of the primer layer are to protect the substrate from corrosion and ensure good adhesion to the metal surface. The middle layer is generally designed to confer thickness to the coating system and hinder the transport of aggressive species to the surface of the substrate, and, finally, the finishing layer that is exposed to the outside provides the surface with the required color and shine.
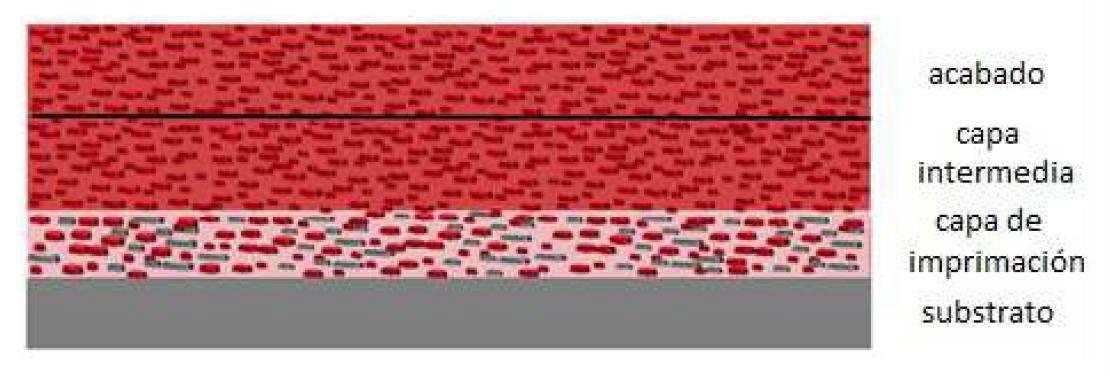
Figure 1: Example of a typical multilayer anti-corrosion coating
Corrosion protection by barrier effect
There are three basic protection mechanisms by which an anti-corrosion paint protects a metal surface against corrosion [3]. One of them is electrochemical protection, either by passivating the surface of the substrate or by the presence of inhibitory pigments that delay electrochemical corrosion reactions.
Another of the protection mechanisms is through the presence of a sacrificial anode or galvanic effect (for example anticorrosive paints rich in zinc). And finally we have the protection by barrier effect [4], which is obtained by applying a coating with low permeability that hinders the diffusion of aggressive species (oxygen, water and ions) through the paint film, and therefore makes it difficult for these corrosive agents to come into contact with the metal substrate. This barrier protection is critical in order to achieve good anti-corrosion performance [4].
Barrier protection is, to some extent, offered by any particle that is impervious to corrosive species. However, some pigments are specially designed to impart these barrier properties by decreasing the permeability of the paint film. They are typically pigments with a laminar shape, which have the ability to orient themselves parallel to the surface of the substrate causing corrosive agents to encounter an intricate diffusion path, and, therefore, reduce the corrosion rate of the substrate (see Figure 2).
In pigmented anticorrosive paints with equidimensional or spherical particles, corrosive agents can migrate much more easily through the coating, as they obstruct much more ineffectively the transport of aggressive species than laminar pigments. In addition, laminar pigments may have a reinforcing effect on the mechanical properties of anticorrosive paint [5]-
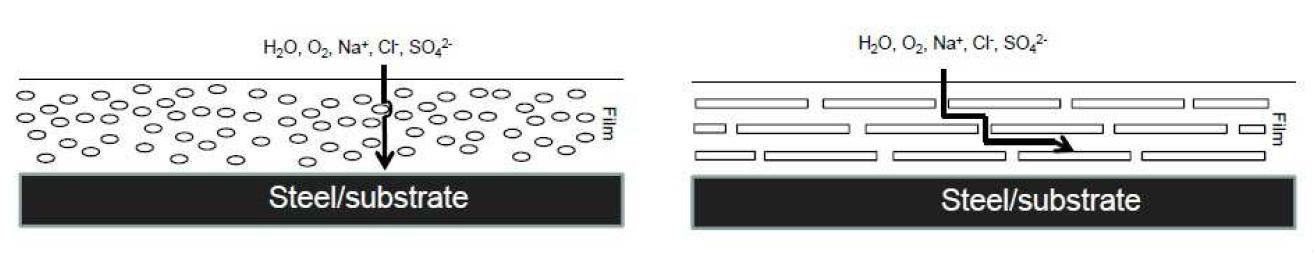
Figure 2: Idealized scheme of the barrier effect caused by laminar pigments
Laminar iron oxides as anticorrosive pigments
Iron oxides occupy a preferential place as pigments or "barrier" agents in the formulation of anticorrosive paints. The widespread use of iron oxides in the sector of anticorrosive paints is due to the fact that they have a series of attributes that make them ideal candidates to be used as protective pigments.
They are compounds with exceptional stability, both chemical and physical, since they resist the attack of acids and bases, ultraviolet radiation and present a considerable thermal resistance. They have a high tolerance to the formulation, being compatible with a wide variety of resins and additives. They are able to minimize the effect of "microfogging" [6] and present an excellent packaging of the particles, minimizing the permeability of the pigment/binder interface.
It should also be noted that iron oxides are not toxic or harmful to the environment, which, taking into account the legal restrictions imposed on the use of traditional anticorrosive pigments, has considerably increased their use.
Among the different varieties of iron oxides on the market, the most used in the formulation of anticorrosive paints are hematites or red iron oxide (α-Fe2O3) of natural origin [7]. In addition to the fact that because natural iron oxides are relatively cheaper than synthetic ones, the use of natural grades is mainly due to the fact that they present a number of technical advantages.
The first of these is due to the different conditions of formation of synthetic and natural hematites, so while natural red oxides commonly have laminar structure, synthetic oxides have equidimensional particle shapes (Figure 3). This different morphology is caused by the different conditions of pressure and temperature of formation, and, mainly, to the different times of crystallization, which in the case of natural ones is billions of years and leads to the formation of laminar particles. Figure 4 shows SEM (scanning electron microscope) images of synthetic and natural oxide hematites, where the different particle morphologies can be clearly observed.
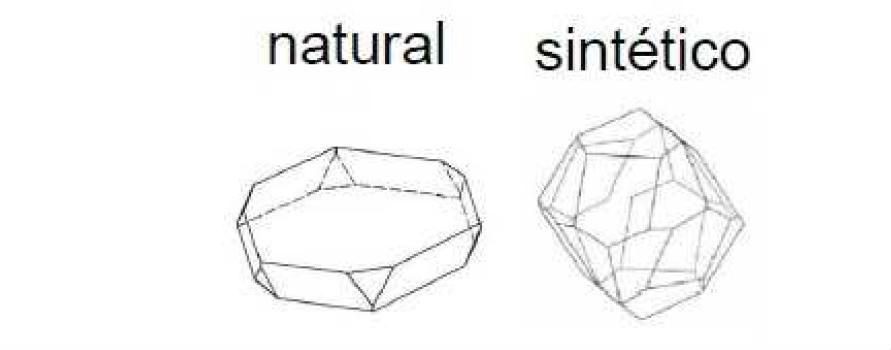
Figure 3: Different morphology of α-Fe2O3 particles according to their synthetic or natural origin
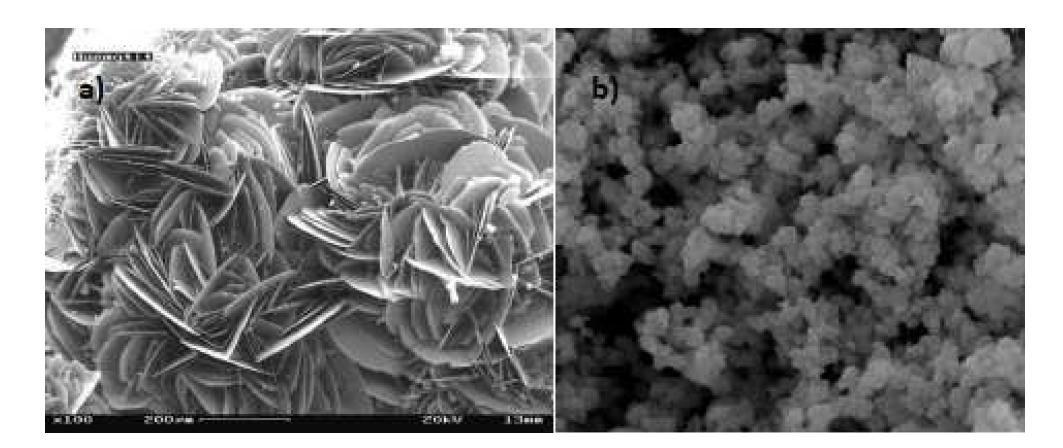
Figure 4. SEM images: a) natural red iron oxide and b) synthetic red iron oxide
The different particle shape has a decisive influence on the performance of the pigment as a "barrier agent", as detailed in the previous section (see Figure 2). Therefore, it is evident that a laminar iron oxide provides a corrosion protection far superior to a "spherical" synthetic oxide. However, not all natural oxides (α-Fe2O3) have the same anticorrosive performance. There are a number of parameters that influence the corrosion protection they provide in a given system.
A decisive factor is the average particle diameter or size, as it has been shown that smaller particle sizes of the natural pigment translate into greater corrosion resistance [8]. This observation has been interpreted according to the better dispersibility of the pigment when its size decreases, which leads to the formation of more compact films (less permeable), since the pigment has been distributed more homogeneously.
Also, a smaller particle diameter of iron oxide seems to lead to an improvement in the practical adhesion of the paint film to the substrate, influencing the anticorrosive behavior. Another parameter that seems to be crucial is the "laminarity" of the particle (expressed as the length/thickness ratio) since the greater the same, the greater the resistance to diffusion of the corrosive species of the pigment.
Another decisive factor that determines the behavior of an iron oxide as an anticorrosive pigment is its content in soluble salts, such as chlorides, sulfates, etc. These species act as electrolytes, increasing the conductivity of the medium and dramatically accelerating the speed of corrosion processes. They also cause the formation of "blisters" (blisters).
The appearance of "blistering" is one of the main problems that anticorrosive paints have to face. Blistering is the first visible sign that corrosion protection is insufficient, and eventually blistering can end up causing a total failure of the protection system due to loss of adhesion.
There are several mechanisms that govern the appearance of "blistering", such as swelling of the film, inclusion of gas, etc., but osmotic processes are considered as the main cause for the development of "blisters" or blisters [9]. Osmotic blistering is caused by water-soluble salts present on the surface of the substrate (see Figure 5), and, therefore, it is evident that an anticorrosive pigment must have a low content of soluble salts to present good anticorrosive performance [10].
Synthetic iron oxides are always contaminated with soluble ions, such as chlorides or sulfates, due to the chemical treatment of the raw material for its production. On the contrary, natural oxides (except in exceptional cases) do not present this type of contamination even at the level of traces. Normally, once the ore has been extracted from the deposit, an intense grinding/micronization of the same is carried out to produce the pigment, and, therefore, in the manufacturing process no chemical treatment is carried out.
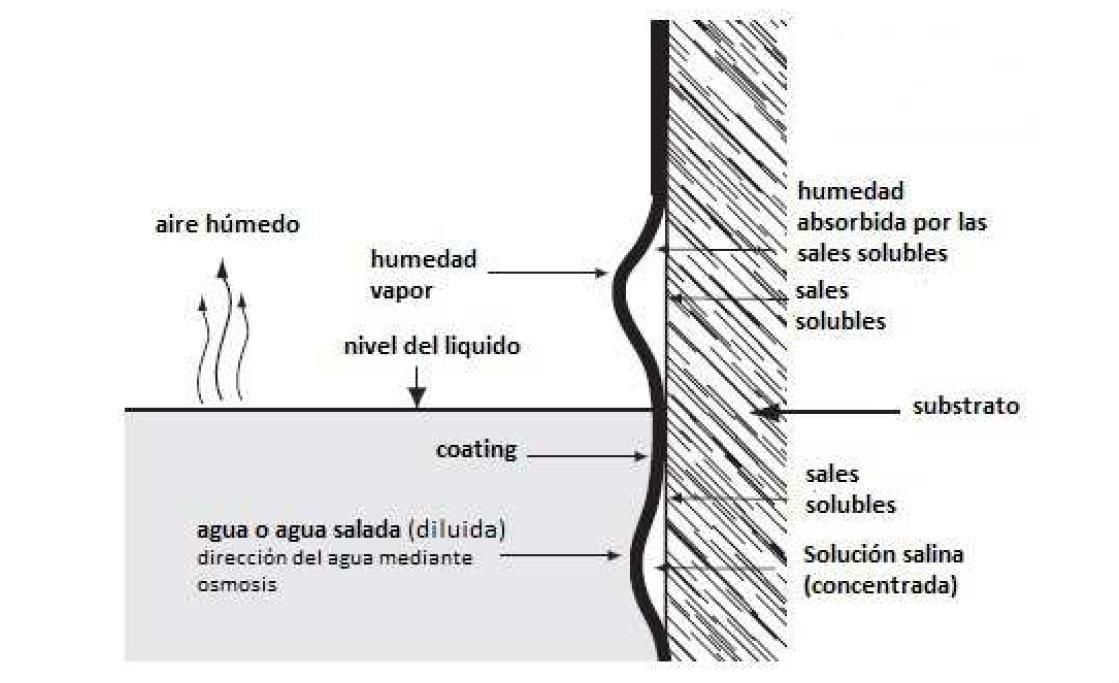
Figure 5: Degradation of anticorrosive paint by osmotic blistering due to the presence of soluble salts [11].
Conclusions
Laminar iron oxides of natural origin are very effective pigments for the formulation of anticorrosive paints. The superior performance of corrosion protection compared to synthetic oxides (see Figure 6) is attributed to a greater barrier effect due to its laminar particle shape, and the absence of soluble salts as contaminants.
Within the natural degrees, the particle size and the degree of laminarity of the same are fundamental parameters to determine the anticorrosive efficacy that the pigment presents.

Figure 6: photographs resulting from the saline test carried out on anticorrosive (alkyd) paints formulated with laminar iron oxide (natural) and equidimensional iron oxide (synthetic). Saline test carried out according to UNE 9227 [12].
References
1) Koch, GH; Brongers, MPH; Thomson, NG, Virmani, YP; Payer; JH. "Corrosion Cost and Preventive Strategies in the United States" Mater. Peform., 65, 1, 2002.
2) Frigate, F, Salai, RP, Armorin, C, Almeida, E. "Compatibility and Incompatibility in Anticorrosive Painting" Prog. Org. Coat., 56, 257, 2006.
3) Sorensen, PA, Kiil, S, Dam-Johansen, K, Weinell, CE. "Anticorrosive coatings: a review", J. Coat. Technol. Res., 135, 6, 2009.
4) Hare, C. Protective "Coatings: Fundamental of Chemistry and Composition". Technology Publishing, Pitysburg 2004.
5) Funke, W. "Towards Enviromentally Acceptable Corrosion Protection by Organic Coating Problems and Realization", J. Coat. Technol., 55, 31, 1983.
6) Ochs, H; Vogelsang, J. "Effect of temperature cycles on impedance spectra of barrier
coatings under immersion conditions", Electrochimica Acta, 49, 2004.
(7) (a) Carter, E. "Recent Developments in Micaceous Iron Oxide Coatings", J. Oil. Color Chem. Assoc., 69, 100, 1986. (b) Wiktorek, S. "Micaceous Iron Oxide in protective Coatings", J. Oil. Color Chem. Assoc., 66, 164, 1983 c) Wiktorek, S. "The orientation of Micaceous Iron Oxide Particle in Organic Coating Applied to Edges", J. Oil. Color Chem. Assoc., 69, 172, 1986.
(8) (a) Giudice C. "Adhesion of LamellarIron Oxide/vinyl Coatings", EuroCoat 292, 5, 1994. (b) Guidice, C; Benitez, JC. "Optimizing the Corrosion Protective Abilities of Lamellar Iron Oxide Containing Primers", Anti-Corros. Meth. Mater., 47, 226, 2000.
9) Funke, W. "Blistering of Paints Films and Filiform Corrosion", Prog. Org. Coat., 9, 29, 1981.
10)De la Fuente, D, Bohm, M, Houyoux, C, Rohwerder, M, Morcillo, M. "The settling of Critical Levels of Soluble Salts for Painting", Prog. Org. Coat., 58, 23, 2007.
11) Figure adapted from: Munger, GC, "Causes and prevention of paint failures", vol 1, chapter 23.
12) Tests carried out by Promindsa.
* Luciano Cuesta Orcoyen holds a PhD in chemical sciences. He is currently the R&D&I director of the company Promindsa (mineral products for industry), located in Zaragoza, Spain. E-mail: [email protected] - Phone: +43 663737878.























Leave your comment