 In the present work, the first results from the study of the corrosion inhibitory behavior of steel presented by potassium sorbate in different saline and corrosive media are presented.
In the present work, the first results from the study of the corrosion inhibitory behavior of steel presented by potassium sorbate in different saline and corrosive media are presented.
by Dr. Guillermo Blustein*
In the present work, the corrosion inhibitory properties of potassium sorbate were studied on SAE-1010 steel electrodes in different corrosive media (NaCl 0.5M, Na2SO4 0.5 M and artificial seawater) using electrochemical techniques (linear polarization and corrosion potential).
The polarization curves were obtained in a conventional three-electrode cell with a large area platinum counterelectrode and a saturated calomel electrode as a reference. SaE 1010 steel included in Teflon was used as a working electrode, with an exposed area of 0.20 cm2 and polished with 600 emery. The scanning speed was 5 mV.s-1 in all cases. At the same time, the corrosion potential was determined as a function of time for SAE 1010 steel specimens in the different corrosive media.
From the polarization curves it could be determined that the corrosion rate of the steel was significantly reduced by the presence of potassium sorbate. In addition, the degree of protection was increased with the increase in the concentration of sorbate, with the optimal concentration being ~70 mM (1% w/v).
In the same sense, the corrosion potential was displaced towards more positive values in the presence of potassium sorbate indicating again a passive state for the steel in that medium.
Introduction
Since the mid-forties, sorbic acid and its salts, especially those of potassium, sodium and calcium, are used as efficient inhibitors of the growth of microorganisms in the food industry. These compounds are attractive for the corrosion protection of steel and alloys since they not only protect from corrosion, but also meet environmental demands, that is, they are not toxic to human health and are also biodegradable [1].
The first works where potassium sorbate is used as a corrosion inhibitor are recent and fundamentally oriented to the protection of copper [2, 3].
In the present work, the first results from the study of the corrosion inhibitory behavior of steel presented by potassium sorbate in different saline and corrosive media are presented. For this, SAE 1010 steel was used, which is the most widely used in the manufacture of metal enclosures and the so-called white line of household appliances (e.g. refrigerators, washing machines, etc.), etc.
Experimental part
Tafel polarization curves were obtained in a conventional three-electrode cell with a large area platinum counterelectrode and a saturated calomel electrode (ECS) as a reference. As a working electrode, a SAE 1010 steel electrode included in Teflon was used, with an exposed area of 0.20 cm2 and polished with emery 600.
The electrolytes used were NaCl 0.5M, Na2SO4 0.5 M and artificial seawater. Different concentrations of potassium sorbate (10-3, 10-2 and 10-1M) were tested with each electrolyte. The sweep range was +250 mV with respect to the open circuit potential and the sweep speed was, in all cases, 5 mV.s-1. The measurements were performed with a Potentiostat/Galvanostat model 273 A from EG&G Princeton Applied Research (PAR) and analyzed with The Softcorr II corrosion software.
The linear polarization curves were made after four hours of immersion of the electrodes in the different electrolytic media used.
The corrosion current densities were obtained from the intersection of the anodic and cathodic branches, which provided results consistent with those obtained using the calculation algorithm provided by the corrosion software used.
The corrosion potential (Ecorr) of electrodes similar to those previously used was monitored at the same immersion times using a high impedance voltmeter.
Results and discussion
The results of both the corrosion current density and the corrosion potential of steel in different electrolytes and different concentrations of potassium sorbate are shown in Table 1.
Table 1: Corrosion potential and corrosion rate of SAE 1010 steel electrodes in different electrolytic media and at different concentrations.
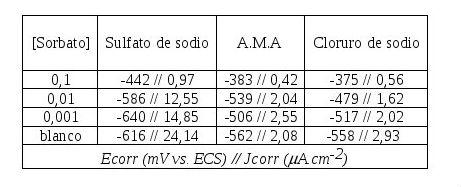
As a general rule, corrosion potentials are shifted towards more positive values than that corresponding to steel in the supporting electrolyte (Table 1). Anyway it can also be seen that steel actually takes on a passive state only when the sorbate concentration is equal to 0.1M.
The values of the corrosion current density are reduced in all cases in the presence of potassium sorbate with respect to them in the supporting electrolyte (Table 1, Figures 1-3). These values depend on the electrolytic medium, so for example the corrosion rate is reduced to an order of magnitude in sodium chloride and artificial seawater, however in sodium sulfate the reduction reaches two orders of magnitude.
In the Tafel polarization curves it can be noted that there is, in the presence of a concentration of potassium sorbate 0.1M and in the three electrolytes, a zone of passivity that is most noticeable in sodium chloride and extends about 200 mV.
In addition, a close observation of the polarization curves in sodium sulfate and artificial seawater shows that with reduced concentrations of inhibitor the steel is more active than in the supporting electrolyte, causing passive zones and zones of active dissolution. The latter was demonstrated by visual inspection of the electrodes after sweeping the linear polarization curves, where passive zones and areas with localized corrosion appeared.
Conclusion
The current and corrosion potential values obtained clearly show that potassium sorbate inhibits corrosion of SAE 1010 steel when the concentration is 0.1M. This brief communication attempts to give a new perspective of application for a molecule that is absolutely compatible with the environment and human health. The mechanism of inhibition is currently the subject of study.
References
[1] Biodegradation 2 (1991) 33-41.
[2] Electrochemical and Solid-State Letters, 9 (2006) B5-B7.
[3] Electrochimica Acta 52 (2007) 1975-1982.























Leave your comment