 Next we will see how from rheology some concepts are clarified in paints and coatings.
Next we will see how from rheology some concepts are clarified in paints and coatings.
by Julián Restrepo*
Dear reader, this paper aims to give a brief review of some concepts of the science of rheology and its application by the formulator of coatings and other people linked to this world, making an approach, perhaps, unconventional, trying to clarify some concepts that, in many cases, are confusing for various formulators. Of course, the topic deserves greater extension, so the objectives of the present are limited to making this writing of an illustrative type.
Overview
Rheology is the science of studying materials, which can be summarized in two words: flow and deformation. But more precisely, it can be defined as the discipline that is dedicated to the study of how materials deform and flow under the action of external forces. This is how the understanding of its principles is very important in various industries, such as plastics, food, printing inks, detergents, greases and lubricating oils, among others [1], and being, of course, essential in the formulation of modern paints and coatings [2]. 1
At this initial point, it is important to homologate the concepts of density, viscosity and surface tension, to establish which of them are involved in the science of rheology, which are defined, briefly, as follows:
Density (represented by the symbol ρ, "ro" in the Greek alphabet), is the magnitude that expresses the relationship that exists between the mass and volume of a substance.
Surface tension (represented by the symbol γ, "gamma"), is the amount of energy needed to increase the surface area per unit area of a substance.
Viscosity (represented by the symbol η, "eta")2, is the property that a substance has by which it tends to oppose its flow (resistance to flow) when a force is applied to it.
Analysis of some paradigms
Based on the above definitions, we observe that only viscosity involves the term fluidity. But then, why are these terms usually used inaccurately, even in some cases they are confused? For this purpose, for illustrative purposes we can analyze Table 1, which reports some variables of various substances, and allows us to analyze some situations.
Table 1: Density, viscosity and surface tension variables for various substances at 25ºC [3] 3
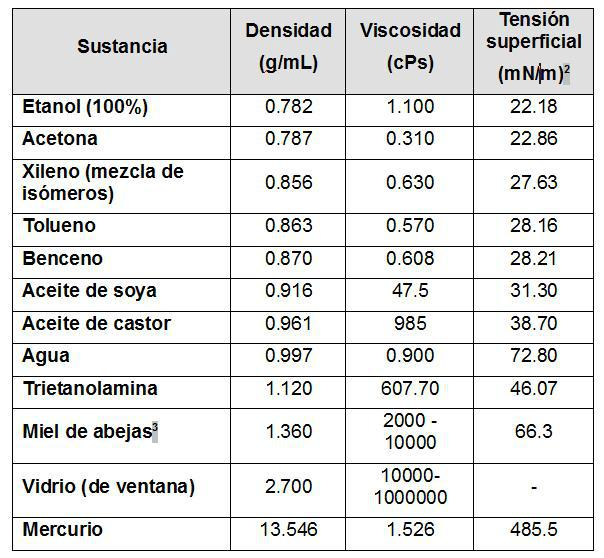
i) The main confusion between the terms density and viscosity arises from the fact that in many of the dictionaries of the Spanish language the term dense appears as a synonym for viscosity [4], although in principle there is no direct relationship between both properties.
Thus, taking liquid water as a reference, there are materials such as mercury, which have a high density but have a low viscosity; for its part, castor oil has a lower density than liquid water, but has a higher viscosity; in the case of honey, it has both a higher density and viscosity than liquid water.
In fact, from an Internet search I observed the following comment: "The density of a paint or a varnish has a lot to do with the pressure effort of the applicator, whether with brushes, rollers, sprays, etc. and how quickly the coating is distributed on the surface at this pressure" [5]... Actually, we must indicate in this case, that both density and surface tension are properties that do not depend on shear stress, while viscosity does, or better, that substances that move away from Newtonian behavior have a rheological profile, with which, as their shear effort increases, their viscosity varies (it can increase or decrease, as we will see later). Therefore, in this situation we have yet another example of the confusion with the terms density and viscosity.
ii) To try to analyze the possible relationships between these variables, we know, for example, that surface tension and density are related by considering the so-called Jurín's law, by which the surface tension of a liquid can be determined, by measuring the height of a column of said liquid in a capillary of known diameter, assuming that the angle of contact θ of the liquids with the capillary wall is small (so that cosθ ≈(1)6
Equation 1
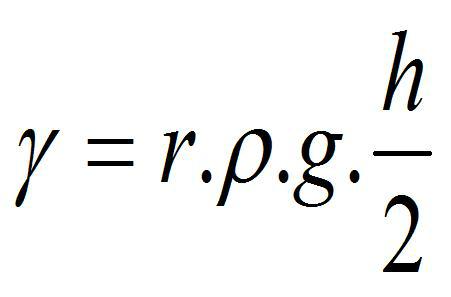
where,
r: Radius of the capillary
d: density of the liquid
g: gravity constant (9.8 m/sec2)
h: Capillary height
From equation 1, it is observed that the density and surface tension are directly proportional.
On the other hand, according to Rodenbush et al. [6], for the case of vegetable oils, the relationship between viscosity and density is defined by the expression:
Equation 2
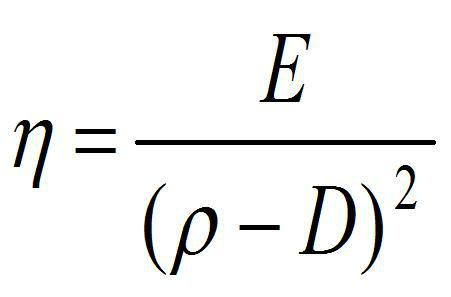
where,
d: density of the liquid
E and D, constants associated with each vegetable oil
From equation (2), it can be seen that, at least for the case of most vegetable oils, the density and viscosity are inversely proportional. But when looking at the data reported in Table 1, it is observed that this is not met for the two oils reported, which indicates the complex relationship between both properties for substances of the same nature, and which is even more complex, considering substances of a nature as dissimilar as those reported.
iii) A very practical way to analyze the relationship between the properties of the substances in Table 1 is to represent them graphically, for example, as a function of density. But to do this, we will choose a group of substances of a similar nature, such as the group of solvents reported. Here's how we get Figure 1 (soybean and beaver oils, triethanolamine, honey, glass, and mercury have not been included):
Figure 1: Graphical representation of viscosity and surface tension against density of the different solvents reported in Table 1.
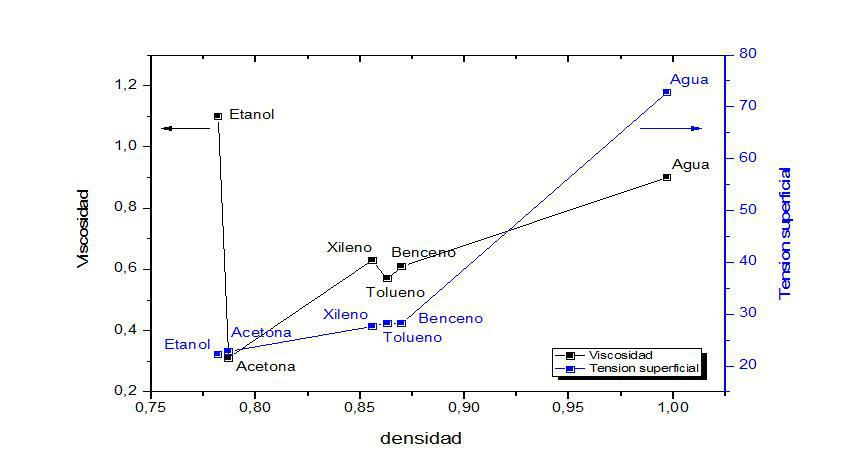
In this figure it is observed that, in general, for solvents, as the density increases, viscosity and surface tension increase together, but this rule cannot be applied in general for any liquid substance.
iv) Finally, we must point out that, referring to the high viscosity of a substance, for example an oil, as "this oil is viscous", is incorrect. We have already seen that liquid materials have an associated viscosity value, therefore, liquids of lower viscosity such as alcohol or water, can also be called "viscous". Strictly speaking, the correct expression in this case would be, this oil has high viscosity. Although at this point we must bear in mind that we are not confusing one property with another, for example, surface tension with viscosity, where our senses would lead us to a methodical doubt (as Descartes once proposed, in the seventeenth century [7]).
Definition of viscosity, from rheology
Complementing the initial definition of viscosity, from the point of view of rheology, it is defined as the relationship between shear stress (τ, "tau") and shear velocity (γ, "gamma point") in Newton's law:
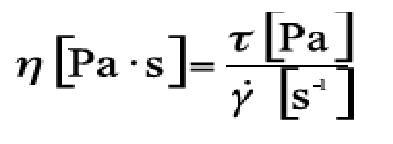
1 Pa.s = 1000 mPa.s = 1000 cP (centiPoises)
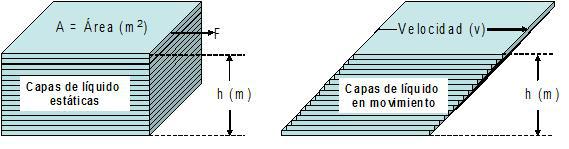
To define the variables shear stress (τ) and shear velocity ( y ), a simple theoretical model is usually used, in which a substance moves under laminar flow conditions between two parallel plates (distance between them = h), and it is assumed that the substance is formed by very thin layers (at the molecular level), as illustrated in Figure 2.
Figure 2: Left, portion of liquid represented in the form of static thin layers; right, the same portion of liquid under laminar flow.
Thus, shear stress (τ) is defined as the ratio of the force applied (F) on the substance, per unit area, being therefore:
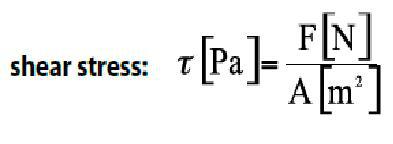
Shear effort,
Figure 2 shows that a force (F) acts on the surface of area substance (A) in the upper plate, while the lower plate does not move (it is stationary), so this force causes a successive movement of the thin layers of the substance (thickness h) between the plates, under conditions of laminar regime. The resulting velocity (v) depends on the internal friction (resistance) between the layers and gradually decreases to zero, in the lowest layer.
The relationship between the difference in velocity (Δv) between two layers and the difference in the thickness of the layers (Δd), which is constant, can be expressed as the shear rate (rate of deformation of the material):
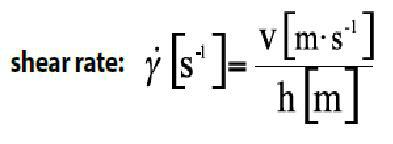
Cutting speed,
Final comment
Some of the most important concepts related to the science of rheology have been discussed and presented very briefly, but we must recognize that other equally important concepts have escaped the objectives of this writing, such as: the definition of yield value, the classification of rheological behaviors (viscosity profiles), the consideration of the time scale in which the deformation is applied to the fluid under study (the concept of the Deborah number, De), which together, allow to have a broader vision of the importance of the science of rheology and much more, in the formulation of current coatings.
References
[1] Introduction to Rheology: http://es.scribd.com/doc/100704088/r53208
[2] Rheology Handbook: Elementis Specialties, A practical guide to rheological additives. 30th anniversary edition, 2008; (www.elementis.com)
[3] (a) Flick, E.W. (1998). Industrial Solvents Handbook (5th Edition); William Andrew Publishing/Noyes; Table 2.140: Texaco Chemical Solvents; (b) http://deconceptos.com/ciencias-naturales/viscosidad; c) Koshkin N.I., Shirkevich M.G. Manual of Physics, Mir Publishing House (1975)
[4] (a) http://www.wordreference.com/sinonimos/viscoso; (b)http://diccionarios.elmundo.es/ dictionaries/cgi/dictionary/lee_diccionario
[5] http://www.sbnprensatecnica.com/noticia/526/Industria-de-la-Pintura/.html
[6] Rodenbush CM, Hsieh FH, Viswanath DS. Density and viscosity of vegetable oils. J Am Oil Chem Soc 1999;76:1415e9
[7] http://es.wikipedia.org/wiki/Ren%C3%A9_Descartes
Footer
1. The adjective "modern" is multiform, but should be interpreted in this context, considering current developments as of the date of this publication.
2. In some texts, this property is still denoted by the symbol μ, but in reality this is reserved for chemical potential.
3. The substances reported are presented according to the density value, in ascending order.
4. Data in relation to air.
5. Properties for common honey with a moisture content between 14-20%. Strictly speaking, in this case reference is made to "ordinary honey", which is obtained in the supermarket, since the characteristics of honey depend on multiple factors, such as its sugar content, humidity, source, type of bee, time of year, etc., etc.
6. Jurin's law is complete
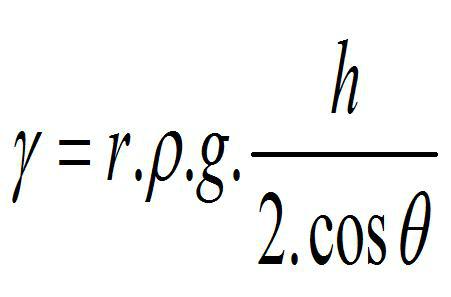
* M.Sc. Ph.D. Julián A. Restrepo R., Factory Service of PPG Industries Colombia, [email protected]























Leave your comment