 An in-depth analysis of the role of surfactants in the paint industry.
An in-depth analysis of the role of surfactants in the paint industry.
by José Tomás Rojas*
One of the most important objectives that paint formulators must achieve, and formulators in the chemical industry in general, is to achieve the compatibility of materials that are by nature incompatible, and must also achieve that this compatibility remains stable over time.
What is compatibility?
In a very general way, we can say that compatibility is the ability of a substance to combine or mix with another, this referring to a physical context, rather than a chemical one. The clearest evidence of good compatibility, especially in the case of liquids, is that the resulting mixture generates a homogeneous solution.
Incompatibility, on the other hand, implies the inability of two substances to combine with each other. The evidence of incompatibility, in general, is a heterogeneous mixture, where the components of the mixture can be identified separately. The mixture of water and oil is the classic example.
Why does incompatibility occur?
As we know, all substances are made up of atoms and molecules. We also know that some substances have a configuration of atoms and molecules that show similarities among themselves, either because of the type of elements that constitute it, either because of the length of the chain, or because of the polarity they demonstrate.
Another thing is that substances possess a stable energy arrangement, which is a product of how their elements are arranged in space. According to this, one substance will be combined with another, as long as the resulting energy situation is more favorable than the energy situation of the individual substance. If the resulting energy situation is unfavorable, there will be no combination.
Surface tension
If we could see inside the molecules that make up a substance, we would find that each of them is surrounded by a certain number of neighboring molecules, which generates a favorable energy exchange. (See Figure 1.)
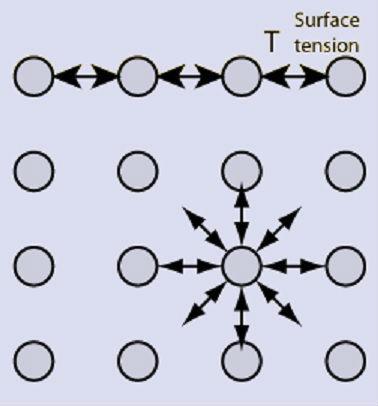
Figure 1. Molecular interaction.
This favorable interaction only occurs within the substance. On the surface, there is a space where there are fewer molecules with which to interact. To balance the unfavorable energy situation that this situation generates, the molecules from the outside tend to contract inward, generating a certain tension, known as Surface Tension (TS).
This stress is a measurable value, typical of liquids, dependent on the chemical structure of the substance and particular to each material. It explains interesting things, such as the spherical shape of the water droplets, which are like this, since this geometric figure is the one that occupies the largest volume with less surface area exposed.
It also allows us to conclude that liquids that have similar surface tension must have a tendency to combine, as well as similarities in their structure that allow an appropriate energy interrelationship.
Dissimilar structures will prefer to separate from each other, narrowing the interaction with their neighbors, and the difference between their TS values will be important. For those who want to elaborate more on the subject, there is a lot of literature about it.
Surfactants
Surfactants are additives, which acting on the surface of the materials, allow incompatible materials to be compatible. For this they have in their structure, an end that in general is compatible with water or with polar substances (Hydrophilic), and another end that in general is compatible with organic or non-polar substances, familiar to oil (lipolyphilic). A very simple explanation of the mechanism of compatibility can be seen in Figure 2.
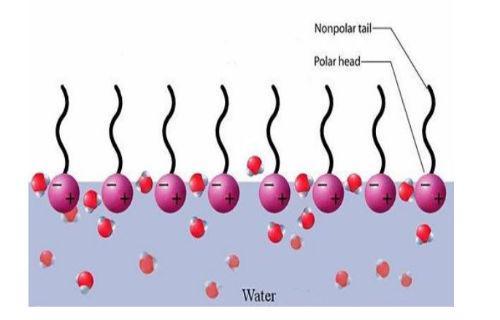
Figure 2. Compatibility mechanism.
By being placed in the middle of two incompatible substances, the surfactants go to the interface between them, orienting their ends to where it locates the best chemical affinity, functioning as a bridge that allows the combination of both materials. One evidence of the effectiveness of a surfactant is its ability to decrease the differences between the TS of two incompatible materials. For example, the TS of water is 72 dynes/cm. When treated with a surfactant, it drops to 35 dynes/cm.
Types of surfactants
In general, surfactants are classified as Ionic and non-ionic. Ionic surfactants are in turn classified as Cationic or Anionic. We also have amphoteric surfactants, whose characteristic is that they have a double polarity. See Figure 3.
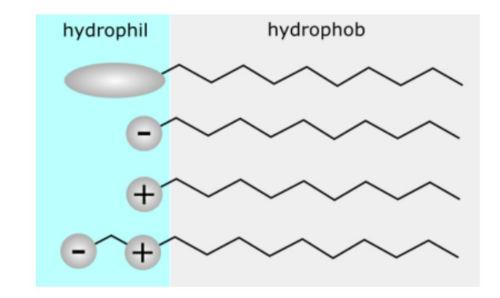
Figure 3. Types of Surfactants
There is a value that characterizes surfactants, which is called Lipophilic Hydrophilic Balance (HLB), which allows to determine how the behavior of the surfactant will be before a mixture of incompatible materials.
For those who wish to do more research on the subject, the Laboratory of Interfacial Phenomena of the University of the Andes, in Venezuela, has a lot of literature available. I leave the link (http://www.firp.ula.ve/archivos/cuadernos/S300A.pdf).
Surfactants in the paint industry
Although they are not known as such in the paint industry, a large number of surfactants are used in it. They are classified more by function than by their structure. Among the best known surfactants we have Dispersants, Humectants, Antifoamers and Leveling Agents, and they are used in both water-based paints and solvent-based paints.
Of course, the use in water-based paints is massive, since here it is about keeping stable a complex combination of materials, of a dissimilar chemical nature, such as organic and inorganic pigments, latex resins, thickening agents, in combination with water. A well-known example of surfactant in industry is Ethoxylated Nonyl Phenol, a non-ionic surfactant, which is used as a moisturizer.
See Figure 4.
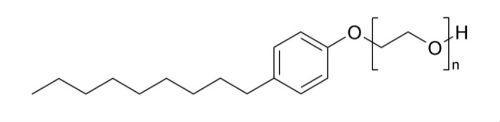
Figure 4. Nonyl Phenol Ethoxylated. The lipophilic part is clearly noticeable, and the hydrophilic part.
The amounts of surfactants used are usually small, approximately 0.5 to 2%. Its efficiency will depend on the correct selection of the surfactant, depending on the system that is to be compatible.
Additionally, surfactants work in a very tight range, which means that adding excess or defective amounts of them to the formula can be very detrimental to their performance. The most critical case is that of silicone-based leveling agents, which, added at recommended doses, correct surface defects such as craters and pin holes in the film. However, an excess of them can cause more craters to appear, impairing the appearance, and even affecting other properties of the film.
* José Tomás Rojas. JTROJAS Pinturas, F.P. You can send your comments to the email [email protected]
























Leave your comment